Pain is a ubiquitous and multifaceted human experience. It functions both as a protective mechanism—warning the organism of potential or actual tissue damage—and as a subjective phenomenon shaped by cognitive, emotional, and social contexts. Over the past century, our understanding of pain has evolved from simplistic stimulus-response models to sophisticated biopsychosocial frameworks. Among the foundational theoretical contributions in pain science is theGate-Control Theory of Pain, introduced in 1965 by Ronald Melzack and Patrick Wall. This theory marked a paradigm shift by proposing that pain perception is not a direct consequence of nociceptor activation alone, but instead is modulated by dynamic interactions among peripheral inputs, spinal gating mechanisms, and descending influences from the brain.
This post reviews the Gate-Control Theory in detail: its historical origins, core concepts and mechanisms, empirical support and limitations, clinical implications, and its role within contemporary models of pain. The aim is to present a rigorous, integrative account suitable for clinicians, researchers, students, and informed readers seeking a deeper understanding of how the nervous system processes nociceptive information.
Historical Context and Motivation
Prior to the Gate-Control Theory, predominant views of pain were largely anatomical and linear: noxious stimuli activated peripheral nociceptors, which transmitted signals via afferent fibers to the spinal cord and onward to brain structures where pain was experienced. This “sensory-deficit” model had difficulty explaining numerous clinical and experimental observations, including:
- Variability in pain experience among individuals exposed to similar injuries.
- Modulation of pain by psychological factors such as attention, expectation, and emotion.
- Phenomena such as phantom limb pain, where pain is perceived in an absent limb.
- Analgesic effects of non-noxious stimulation (e.g., rubbing a bumped elbow reduces pain).
- The effectiveness of counterirritation and transcutaneous electrical nerve stimulation (TENS).
Melzack and Wall synthesized anatomical, physiological, and psychophysical evidence into a model that could account for these complexities. Their central proposition was that a “gate” within the dorsal horn of the spinal cord regulates the transmission of nociceptive signals to higher centers. This mechanical metaphor—like a gate that opens or closes—offered a parsimonious way to integrate peripheral and central modulatory influences.
Core Concepts of the Gate-Control Theory

The Gate-Control Theory rests on several interrelated principles and components:
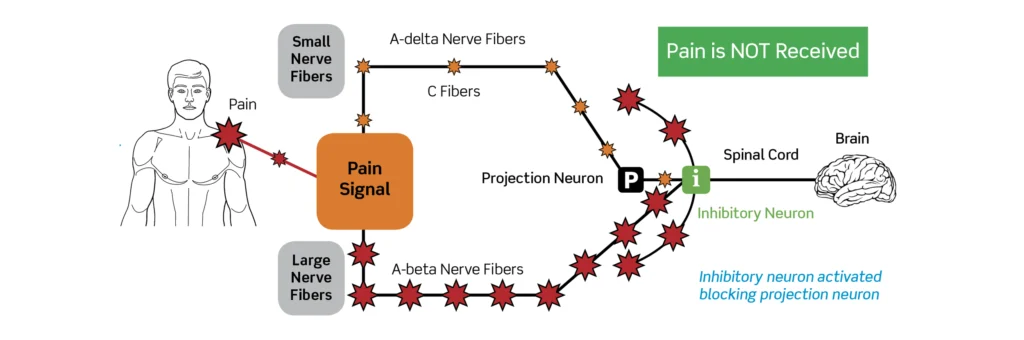
- Peripheral Afferent Fibers:
- Large-diameter fibers (Aβ): Myelinated, fast-conducting fibers that primarily convey non-nociceptive mechanoreceptive input such as touch, pressure, and vibration.
- Small-diameter fibers (Aδ and C): Thinly myelinated (Aδ) and unmyelinated (C) fibers that convey noxious and thermal stimuli, producing fast sharp pain (Aδ) and slower, burning pain (C).
- Spinal “Gate” in the Dorsal Horn:
- The dorsal horn of the spinal cord contains interneurons and projection neurons that act as a local integrative network. According to the theory, the relative activity of large and small fibers determines the state of a neural “gate” that modulates ascending transmission.
- Increased activity of large-diameter fibers tends to close the gate, inhibiting transmission of nociceptive signals; increased activity of small-diameter fibers tends to open the gate, facilitating transmission.
- Transmission (T) Cells:
- Projection neurons within the dorsal horn (termed T cells in the original formulation) relay signals from the spinal cord to higher centers such as the thalamus and cortex. The output of T cells is influenced by the gating mechanism.
- Central Control Mechanisms:
- The brain exerts descending influences that can either open or close the gate by modulating interneurons and projection neurons in the spinal cord. Cognitive and emotional factors (attention, expectation, mood) can alter descending inputs and thereby modulate pain perception.
- Psychological Modulation:
- The theory explicitly integrates psychological variables, predicting that attention, arousal, and other mental states can influence pain by altering central control signals.
These elements form a dynamic system: the net perceived pain results from the balance of peripheral nociceptive input, non-nociceptive input, local spinal processing, and descending modulation from supraspinal centers.
Mechanistic Details and Neural Substrates
Although initially framed with somewhat schematic wiring diagrams, subsequent neurophysiological research has mapped many elements of the Gate-Control Theory onto identified cellular and synaptic mechanisms.
- Inhibitory interneurons in the dorsal horn: GABAergic and glycinergic interneurons are key mediators of presynaptic and postsynaptic inhibition. Activation of large-fiber afferents can recruit inhibitory interneurons that suppress transmission from nociceptive afferents to projection neurons.
- Primary afferent depolarization and presynaptic inhibition: Large-fiber activity can produce presynaptic inhibition of small-fiber terminals, decreasing neurotransmitter release from nociceptors.
- Ascending projection pathways: Spinothalamic and spinoreticular tracts carry nociceptive information to the thalamus and brainstem, where further modulation occurs.
- Descending pain modulatory systems: Brainstem nuclei such as the periaqueductal gray (PAG) and rostroventromedial medulla (RVM) project to the dorsal horn and can facilitate or inhibit nociceptive transmission. Neurotransmitters implicated include endogenous opioids, noradrenaline, and serotonin.
- Plasticity and sensitization: Persistent nociceptive activity can induce synaptic plasticity within dorsal horn circuits (central sensitization), altering the gating dynamics and producing hyperalgesia and allodynia.
Thus, the gate concept corresponds to an ensemble of inhibitory and excitatory synaptic interactions rather than a single anatomical structure. Importantly, the gating process operates at multiple temporal and spatial scales—ranging from instantaneous presynaptic inhibition to long-term changes resulting from injury and learning.
Empirical Support
The Gate-Control Theory stimulated a large body of experimental and clinical research. Notable findings that align with its predictions include:
- Analgesic effect of non-nociceptive stimulation: Touch and vibration, primarily transmitted by large Aβ fibers, can reduce pain—consistent with gate closure mediated by large-fiber activity. Clinically, rubbing a painful site, applying cold or pressure, or using TENS often produce analgesia.
- Descending modulation: Activation of PAG and RVM circuits produces analgesia in animal models and humans; these effects involve endogenous opioids and descending monoaminergic pathways.
- Psychological influences: Placebo analgesia, distraction, and attentional focus can modulate pain reporting and neural responses in ascending pain pathways, demonstrating top-down control that fits the central control component of the model.
- Presynaptic inhibition: Electrophysiological studies have documented mechanisms by which large-fiber inputs suppress small-fiber neurotransmission in the spinal cord.
- Temporal patterns and wind-up: Repetitive C-fiber stimulation can produce increased excitability in dorsal horn neurons (wind-up), aligning with the theory’s emphasis on dynamic gating and plasticity.
Collectively, these observations provide substantial support for the general idea that spinal and supraspinal mechanisms modulate nociceptive transmission and that non-nociceptive inputs and psychological processes can influence pain.
Limitations and Critiques
While the Gate-Control Theory was revolutionary and remains influential, it also has limitations. Critiques include:
- Oversimplification: The original gate schematic presented a relatively simple binary gating mechanism (open/closed). Contemporary knowledge reveals a far more complex network of cell types, neurotransmitters, and microcircuits in the dorsal horn, producing graded and context-dependent modulation rather than a single gate state.
- Incomplete explanation for chronic pain: While the theory can accommodate phenomena like central sensitization, it does not fully account for the persistent and maladaptive plasticity seen in many chronic pain disorders, where structural, immune, and glial contributions are significant.
- Phantom limb and neuropathic pain complexities: The theory recognized central contributions to phantom pain, but later work exposed complex cortical reorganization, ectopic peripheral activity, and immune-glial interactions that extend beyond the original formulation.
- Molecular and immune mechanisms: Advances in neuroimmunology and molecular biology have revealed roles for microglia, astrocytes, cytokines, and chemokines in modulating nociceptive processing—factors not encompassed in the original gate model.
- Heterogeneity of pain pathways: There is substantial diversity among nociceptors, interneurons, and projection neurons. The original theory’s generalization about “large” versus “small” fibers does not capture the functional heterogeneity uncovered by modern single-cell studies.
These limitations do not invalidate the Gate-Control Theory; rather, they indicate that the theory provides an important but incomplete scaffold that has been enriched and refined by subsequent research.
Integration with Contemporary Pain Models
Modern models of pain—especially the biopsychosocial model and neuromatrix theory—build on and extend the gate concept. Key integrations include:
- Neuromatrix theory: Proposes that pain emerges from distributed networks (a neuromatrix) involving the thalamus, limbic system, somatosensory cortices, and associative cortices. The neuromatrix produces a unified experience of pain influenced by sensory inputs, cognitive evaluations, and affective states. The Gate-Control Theory’s emphasis on modulation at the spinal level complements the neuromatrix’s focus on central network dynamics.
- Biopsychosocial model: Pain is seen as an outcome of biological, psychological, and social factors. Gate-control mechanisms provide a biological substrate through which psychological factors (e.g., expectation, attention, mood) exert influence.
- Predictive processing and Bayesian frameworks: These contemporary theories conceptualize perception, including pain, as the brain’s inference about sensory input based on prior expectations and prediction errors. Descending signals that modulate spinal processing can be interpreted as precision-weighted predictions that shape sensory gain—paralleling the Gate-Control Theory’s central modulation.
Thus, rather than being supplanted, the Gate-Control Theory is best regarded as a foundational element that sits within a richer multilevel understanding of pain.
Clinical Implications
The Gate-Control Theory has had direct and indirect clinical impacts:
- Non-pharmacological interventions: Techniques that stimulate large-diameter afferents—such as TENS, massage, acupuncture (partly via mechanoreceptive stimulation), and vibration—can reduce pain by engaging spinal inhibitory mechanisms.
- Cognitive and behavioral therapies: The recognition that cognition and emotion modulate pain supports psychological therapies, including cognitive-behavioral therapy (CBT), mindfulness-based stress reduction, and acceptance-based approaches, which can alter descending modulatory control.
- Multimodal analgesia: The theory encourages combining peripheral and central approaches (e.g., local anesthetics, systemic analgesics, and psychological strategies) to influence both input and modulation.
- Spinal cord stimulation (SCS): SCS, which applies electrical stimulation to dorsal columns, reduces chronic neuropathic pain for some patients by activating dorsal column fibers and recruiting inhibitory circuits—an application directly inspired by gate-control principles.
- Rehabilitation strategies: Early mobilization and graded exposure to movement can modulate sensory inputs and cognitive factors, influencing gating and central processing to reduce pain-related disability.
Although gate-based approaches provide benefits for many patients, individualized assessment remains crucial. Chronic pain conditions often involve complex peripheral, central, and psychosocial contributors that require tailored multimodal management.
Experimental and Research Directions
The Gate-Control Theory continues to motivate research across several domains:
- Cellular and circuit mapping: Use of transgenic animals, optogenetics, and single-cell transcriptomics to identify interneuron subtypes in the dorsal horn and their roles in inhibition and gating.
- Neuroimmune interactions: Investigating how microglia and astrocytes influence dorsal horn excitability and gating, particularly in chronic pain states.
- Mechanisms of descending control: Elucidating how higher brain regions (prefrontal cortex, amygdala, hypothalamus) interact with brainstem modulatory systems to influence spinal gating, and how psychological interventions reshape these circuits.
- Translation and neuromodulation: Refining neuromodulatory therapies (TENS, SCS, dorsal root ganglion stimulation) to optimize fiber recruitment and inhibitory engagement with fewer side effects.
- Computational models: Developing formal, quantitative models of gating within predictive coding and reinforcement learning frameworks to relate physiological dynamics to subjective reports of pain.
These avenues are not only scientifically compelling but hold promise for the development of targeted therapies for refractory pain conditions.
Conclusion
The Gate-Control Theory of Pain is one of the most consequential models in pain science. By proposing that pain is modulated at the spinal level via an interaction between large- and small-fiber inputs, and by highlighting the role of descending control from the brain, Melzack and Wall provided a framework that reconciled physiological, psychological, and clinical observations. While modern research has revealed a far more intricate network of cellular, molecular, and systemic processes than the original schematic suggested, the core insight—that pain is not a simple direct readout of peripheral nociception, but a product of dynamic modulation—remains central to contemporary understanding.
Clinically, the theory informed practical interventions—from tactile counterstimulation to neuromodulation and cognitive therapies—and continues to underpin multimodal approaches to pain management. Scientifically, it stimulated decades of research into spinal circuitry, descending modulation, and the interplay between peripheral inputs and central processes. Ultimately, the Gate-Control Theory exemplifies how integrative theoretical models can catalyze progress in both basic science and clinical practice, and it retains enduring relevance as we refine our comprehension of one of the most complex and consequential human experiences: pain.
References and Suggested Reading
- Here are the references rewritten in APA 7th edition style:
- Melzack, R., & Wall, P. D. (1965). Pain mechanisms: A new theory. Science, 150(3699), 971–979. https://doi.org/10.1126/science.150.3699.971
- Basbaum, A. I., & Fields, H. L. (1984). Endogenous pain control systems: Brainstem spinal pathways and endorphin circuitry. Annual Review of Neuroscience, 7, 309–338. https://doi.org/10.1146/annurev.ne.07.030184.001521
- Woolf, C. J., & Salter, M. W. (2000). Neuronal plasticity: Increasing the gain in pain. Science, 288(5472), 1765–1768. https://doi.org/10.1126/science.288.5472.1765
- Apkarian, A. V., Baliki, M. N., & Geha, P. Y. (2009). Towards a theory of chronic pain. Progress in Neurobiology, 87(2), 81–97. https://doi.org/10.1016/j.pneurobio.2008.09.018
- Tracey, I., & Bushnell, M. C. (2009). How neuroimaging studies have challenged us to rethink: Is chronic pain a disease? The Journal of Pain, 10(11), 1113–1120. https://doi.org/10.1016/j.jpain.2009.09.014
For detailed experimental and clinical methods, readers are encouraged to consult current review articles and specialty textbooks in pain neuroscience and clinical pain management.
Discover more from Decroly Education Centre - DEDUC
Subscribe to get the latest posts sent to your email.
