The Gate-Control Theory of pain, first articulated by Ronald Melzack and Patrick Wall in 1965, represented a paradigm shift in the understanding of pain mechanisms. Rather than viewing pain as a simple linear transmission of nociceptive signals from peripheral receptors to the brain, the theory proposed an interactive process in which spinal cord mechanisms—functioning as a “gate”—modulate the flow of nociceptive information to higher centers. This in-depth article explores the clinical implications of Gate-Control theory, from TENS and physical therapy to psychological and pharmacological interventions—while addressing modern critiques like neuroplasticity and individual pain variability.

Over the ensuing decades the theory has evolved, been refined by neurophysiological discoveries, and integrated into contemporary pain science. Clinically, the Gate-Control Theory has had profound implications across assessment, treatment, rehabilitation, and health policy. This article reviews the foundations of the theory, summarizes neurophysiological refinements, and explores the major clinical consequences, with emphasis on evidence-based interventions, limitations, and future directions.
Foundations of the Gate-Control Theory
- Core proposition: Neural transmission of pain signals can be facilitated or inhibited at the spinal cord level by the relative activity of different afferent fibers and descending modulatory systems. The dorsal horn of the spinal cord serves as the “gate,” where signals from small-diameter nociceptive fibers (A-delta and C fibers) and large-diameter non-nociceptive fibers (A-beta) converge, and interneuronal circuits determine net transmission to projection neurons.
- Key components:
- Small fibers tend to open the gate (promote pain signal transmission).
- Large fibers tend to close the gate (inhibit transmission), explaining why rubbing a bumped area reduces pain.
- Descending pathways from brainstem and cortical centers modulate gate status, integrating cognitive, emotional, and contextual inputs.
- Implications for pain as multidimensional: The theory emphasized that sensory-discriminative, affective-emotional, and cognitive-evaluative components interact to shape pain perception.
Neurophysiological Refinements and Contemporary Understanding
Since the original formulation, extensive research has refined the cellular and molecular mechanisms whereby gating occurs:
- Dorsal horn circuitry: The concept of “gate” is now understood as complex interactions among excitatory and inhibitory interneurons (GABAergic, glycinergic), projection neurons (e.g., wide dynamic range neurons), and primary afferent terminals. Central sensitization, wind-up, and synaptic plasticity alter gating efficacy.
- Descending modulation: Descending pathways from periaqueductal gray (PAG), rostroventromedial medulla (RVM), and other brainstem nuclei exert both inhibitory and facilitatory influences mediated by neurotransmitters (serotonin, noradrenaline, endogenous opioids).
- Glia and neuroinflammation: Microglia and astrocytes release cytokines and neuromodulators that can potentiate or maintain a hyperexcitable dorsal horn state, contributing to chronic pain and altered gating.
- Peripheral and central sensitization: Persistent nociceptive input can enhance synaptic efficacy (long-term potentiation–like mechanisms) and reduce inhibitory tone, leading to hyperalgesia and allodynia—phenomena consistent with maladaptive gating.
- Top-down cognitive and emotional modulation: Functional imaging and psychophysical research confirm that expectation, attention, mood, and learning significantly influence pain experience through descending modulation and cortical control.
These refinements preserve the central idea of modulatory control but replace a simple physics-like “gate” with a dynamic, plastic network spanning peripheral, spinal, and supraspinal levels.
Clinical Implications
The Gate-Control Theory and its contemporary extensions inform many clinical domains: acute pain management, chronic pain treatment, rehabilitation, perioperative care, and psychological interventions. Below are key clinical implications and how they translate into practice.
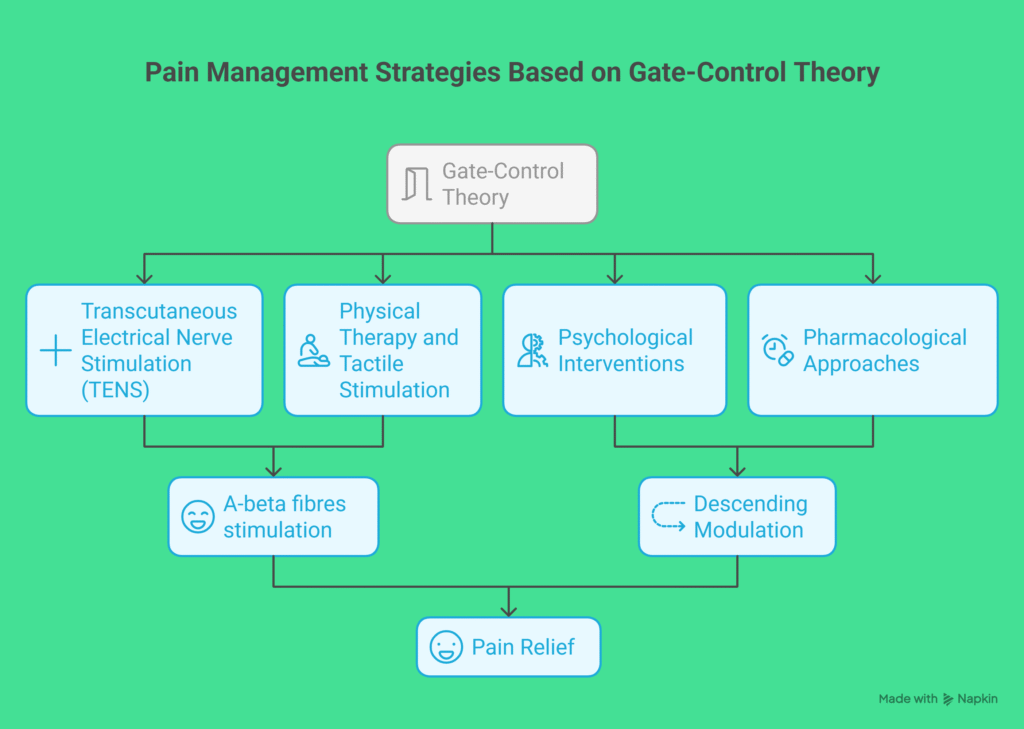
1. Nonpharmacologic, Peripheral Modulation Techniques
- Transcutaneous electrical nerve stimulation (TENS):
- Rationale: Activation of large-diameter afferents to inhibit nociceptive transmission at the dorsal horn.
- Evidence: Mixed but supportive for short-term relief in some musculoskeletal and neuropathic conditions. Best implemented as part of multimodal therapy.
- Clinical use: Low-risk adjunct for acute and chronic pain; individualized parameter titration advised.
- Manual therapies and rubbing/massaging:
- Rationale: Large-fiber mechanical stimulation can transiently decrease pain (exemplified by rubbing a bumped knee).
- Clinical application: Useful in acute injury, rehabilitation, and palliative symptom management; effects often temporary and should be combined with corrective exercise or functional rehabilitation.
- Thermal modalities (ice, heat) and counterirritation:
- Mechanism: Activation of different sensory afferents and local effects on inflammation and conduction.
- Use: Short-term analgesia, often as adjunctive care.
- Spinal cord stimulation (SCS):
- Rationale: Direct modulation of dorsal column activity and dorsal horn circuits via implanted electrodes to reduce transmission of pain signals.
- Indications: Refractory neuropathic pain (failed back surgery syndrome, complex regional pain syndrome), with increasing evidence for specific patient subgroups.
- Outcomes: Substantial pain reduction for selected patients; invasive and costly with surgical and device risks—careful patient selection and trialing are essential.
2. Pharmacologic Approaches Targeting Spinal/Descending Modulation
- Opioids:
- Mechanism: Act at spinal dorsal horn and supraspinal sites to enhance inhibitory tone via mu-opioid receptors; also engage descending inhibitory pathways.
- Clinical role: Effective for acute severe pain and some cancer-related pain; chronic use limited by tolerance, dependence, hyperalgesia, and adverse effects.
- Gate-theory relevance: Supports the concept that modulation at spinal and supraspinal levels reduces pain transmission.
- Adjuvant analgesics (anticonvulsants, antidepressants):
- Mechanisms: Gabapentinoids, tricyclic antidepressants (TCAs), and SNRIs modulate neuronal excitability and enhance descending inhibition (noradrenergic and serotonergic mechanisms), thereby improving gating.
- Indications: Neuropathic pain syndromes and certain chronic pain conditions.
- Clinical implications: Use as first-line agents for many neuropathic pains; therapeutic effect reflects modulation of gating and central sensitization.
- Topical agents:
- Localized modulation of peripheral nociceptors and ion channels (e.g., capsaicin desensitization, lidocaine blockade) reduces nociceptive input to the gate.
3. Cognitive and Affective Interventions
- Cognitive-behavioral therapy (CBT):
- Rationale: Alters cognitive and emotional appraisal, attention, and coping strategies to modify descending modulatory influences on pain.
- Efficacy: Robust evidence for reducing pain-related disability and improving coping in chronic pain; small-to-moderate effects on pain intensity.
- Clinical integration: Considered a foundational component of multimodal chronic pain management.
- Mindfulness-based stress reduction (MBSR) and acceptance-based therapies:
- Mechanism: Modulate attention and affective responses, influencing top-down control of pain processing.
- Evidence: Beneficial for pain-related psychological outcomes and sometimes for pain intensity; provided as complement to medical and rehabilitative care.
- Placebo and expectancy effects:
- Clinical relevance: Expectations and therapeutic context can recruit endogenous analgesic systems (opioid and non-opioid pathways), demonstrating the potency of cognitive modulation predicted by the Gate-Control framework.
- Implication: Clinician communication, ritual of care, and patient-clinician relationship meaningfully influence outcomes.
4. Rehabilitation and Functional Restoration
- Exercise therapy:
- Mechanisms: Exercise can reduce central sensitization, improve descending inhibitory function, and enhance endogenous opioid and monoaminergic systems. Peripheral benefits (strength, mobility) also reduce nociceptive input.
- Evidence: Effective for many chronic musculoskeletal conditions (low back pain, osteoarthritis), improving pain and function.
- Clinical approach: Graded, individualized exercise programs with attention to fear-avoidance and pacing.
- Graded exposure and activity pacing:
- Rationale: Address maladaptive avoidance driven by pain-related fear (which can exacerbate central facilitation), gradually restoring function and normalizing nociceptive signaling.
- Outcome: Reductions in disability and improved quality of life.
5. Perioperative and Acute Pain Management
- Multimodal analgesia:
- Principle: Combine agents that act at different sites and mechanisms (e.g., NSAIDs, regional anesthesia, acetaminophen, gabapentinoids) to reduce nociceptive input and enhance inhibitory control, thereby minimizing opioid requirements and central sensitization.
- Evidence: Reduces postoperative pain, opioid consumption, and possibly chronic postoperative pain when effectively implemented.
- Regional anesthesia:
- Mechanism: Interrupts peripheral nociceptive input to prevent central sensitization and “opening” of the gate chronically.
- Clinical implications: Appropriate regional techniques can reduce acute pain and may reduce the incidence of chronic postoperative pain.
6. Addressing Chronic Pain and Central Sensitization
- Recognition of central mechanisms:
- Clinical importance: Chronic pain conditions (fibromyalgia, centralized low back pain) often reflect altered central processing and dysfunctional gating rather than ongoing nociceptive tissue damage.
- Diagnostic implications: Emphasis on careful assessment for signs of central sensitization (widespread hyperalgesia, allodynia, disproportionate pain, cognitive-emotional contributions).
- Therapeutic strategies:
- Combine pharmacologic agents targeting central excitability (antidepressants, gabapentinoids) with psychological therapies and exercise.
- Interventions aimed solely at peripheral tissues (repeated surgeries or interventions focused on presumed peripheral nociceptive sources) often fail if central sensitization predominates.
7. Personalized Medicine and Patient Selection
- Heterogeneity in pain mechanisms:
- Not all patients respond similarly to interventions that modulate gating. Phenotyping patients by dominant mechanisms (nociceptive, neuropathic, centralized) improves treatment selection.
- Predictors of response:
- Quantitative sensory testing (QST), pain modulation assessments (conditioned pain modulation), psychological profiling, and biomarkers may help predict response to therapies that rely on gating mechanisms (e.g., SCS, antidepressants).
8. Health Systems and Policy Implications
- Emphasis on multimodal, interdisciplinary care:
- Gate-related insights support integrated programs combining pharmacologic, behavioral, and physical therapies, rather than siloed, procedure-focused approaches.
- Education and prevention:
- Patient and clinician education on pain as a biopsychosocial phenomenon can reduce unnecessary procedures, improve adherence to active therapies, and lower chronicity risk.
- Cost-effectiveness:
- Early multimodal interventions that prevent central sensitization may reduce long-term disability and healthcare costs compared to repeated single-modality treatments.
Limitations and Cautions
- Model simplification: The Gate-Control Theory, while foundational, simplifies the complexity of pain neurobiology. It should be used as a conceptual framework rather than a literal mechanistic map.
- Variable evidence strength: Specific interventions inspired by gating (e.g., TENS) show heterogeneous evidence; they are often modestly effective and best used adjunctively.
- Risk of overattribution: Not all pain phenomena are explained solely by gating; peripheral pathology, structural lesions, and biochemical etiologies must be evaluated and treated appropriately.
- Individual variability: Genetic, psychosocial, and environmental factors modulate response to gate-targeted therapies; one-size-fits-all approaches are inappropriate.
Future Directions
- Advanced neuromodulation: Development of closed-loop spinal cord stimulation, high-frequency and burst stimulation paradigms, and dorsal root ganglion (DRG) stimulation seeks to improve efficacy and specificity of gating-based neuromodulation.
- Biomarkers and phenotyping: Improved tools to identify dominant pain mechanisms (neuroimaging, QST, inflammatory markers) will enable targeted gating-based interventions.
- Combination therapies: Rational combinations of neuromodulation, pharmacology, and cognitive interventions will be tested to synergistically restore inhibitory control.
- Translational research on glia and inflammation: Targeting neuroimmune contributors to dysfunctional gating could yield novel analgesics and disease-modifying approaches for chronic pain.
- Digital therapeutics and remote delivery: Scalable cognitive and behavioral interventions leveraging telemedicine and digital platforms could augment descending control and patient self-management.
Practical Clinical Recommendations
- Adopt a biopsychosocial assessment framework to identify peripheral, spinal, and supraspinal contributors to pain.
- Use multimodal analgesia perioperatively and for acute severe pain to minimize central sensitization.
- Reserve invasive neuromodulation for well-selected patients after appropriate trials and multidisciplinary evaluation.
- Prioritize active rehabilitation (graded exercise, functional restoration) and psychological therapies (CBT, acceptance-based approaches) for chronic pain, particularly when central mechanisms are suspected.
- Employ adjuvant pharmacotherapy (TCAs, SNRIs, gabapentinoids) for neuropathic and centralized pain syndromes, with attention to side effects and long-term monitoring.
- Incorporate patient education about pain modulation and the role of expectation, attention, and mood in shaping pain experience to enhance engagement and outcomes.
Conclusion
The Gate-Control Theory catalyzed a transformation in pain medicine from a strictly biomedical, lesion-focused view to a dynamic, integrative model emphasizing modulation at multiple levels of the nervous system. Clinically, this perspective legitimizes a broad spectrum of interventions—peripheral counterstimulation, pharmacologic modulation, neuromodulation, cognitive therapies, and rehabilitative strategies—designed to restore inhibitory control and reduce maladaptive facilitation.
While the original gate metaphor has been refined into a complex neurobiological framework incorporating synaptic plasticity, glial function, and descending control, its clinical legacy endures: effective pain management requires multimodal, individualized strategies that address the interplay among sensory input, spinal processing, and top-down modulation. Continued translational research and personalized care models promise to refine how gating principles are applied and improve outcomes for patients with acute and chronic pain.
Explore more on Clinical Implications of the Gate-Control Theory
Melzack, R., & Wall, P. D. (1965). Pain mechanisms: A new theory. Science, 150(3699), 971–979. https://doi.org/10.1126/science.150.3699.971
Basbaum, A. I., & Fields, H. L. (1984). Endogenous pain control systems: Brainstem spinal pathways and endorphin circuitry. Annual Review of Neuroscience, 7, 309–338. https://doi.org/10.1146/annurev.ne.07.030184.001521
Moseley, G. L., & Flor, H. (2012). Targeting cortical representations in the treatment of chronic pain: A review. Neurorehabilitation and Neural Repair, 26(6), 646–652. https://doi.org/10.1177/1545968311433209
Apkarian, A. V., Baliki, M. N., & Geha, P. Y. (2009). Towards a theory of chronic pain. Progress in Neurobiology, 87(2), 81–97. https://doi.org/10.1016/j.pneurobio.2008.09.018
Tracey, I., & Bushnell, M. C. (2009). How neuroimaging studies have challenged us to rethink: Is chronic pain a disease? The Journal of Pain, 10(11), 1113–1120. https://doi.org/10.1016/j.jpain.2009.09.014
Katz, J., & Melzack, R. (1990). Pain “memories” in phantom limbs: Review and clinical observations. Pain, 43(3), 319–336. https://doi.org/10.1016/0304-3959(90)90027-G
Flor, H. (2002). Phantom-limb pain: Characteristics, causes, and treatment. The Lancet Neurology, 1(3), 182–189. https://doi.org/10.1016/S1474-4422(02)00074-1
Scheman, J. (2022). Pain and the brain: What is the gate control theory? Cleveland Clinic. https://health.clevelandclinic.org/gate-control-theory-of-pain
Campbell, T. S., Johnson, J. A., & Zernicke, K. A. (2013). Gate control theory of pain. In Encyclopedia of Behavioral Medicine (pp. 832–834). Springer. https://doi.org/10.1007/978-1-4419-1005-9_1134
Discover more from Decroly Education Centre - DEDUC
Subscribe to get the latest posts sent to your email.
